Cryogenic Sample Tracking
Industry Need
Modern life sciences commonly require long term preservation of biological material; cryogenic processing and storage provides a means to this end. With a large quantity of small samples in storage, accurate tracking is critical. Barcoded labels (with linear or 2D symbols) are the preferred method, but the extreme environmental stresses can cause failure with standard media.
Cryogenic conditions have become commonplace in multiple applications, ranging from pharmaceutical processing to lyophilization of vaccines. Cryopreservation of tissues and cell suspensions in small vials provides its own set of challenges, requiring conformability to small diameter surfaces and adhesion to low surface energy plastics such as polypropylene and polyethylene. Increasingly common cryogenic shipping of vaccines, tissues, and medicines in liquid nitrogen with temperatures as low as -112°F /-80°C now subjects a greater range of barcoded items to these stresses.
Continue reading »
Block Out Labeling
Industry Need
Labels are commonly used to convey important information about the products they mark. However, an important element of labeling has become more prevalent: concealing inaccurate or out-of-date information previously in place. These kinds of labels can clearly communicate changed pricing without drawing customer attention, or may enable use of re-tasked or inaccurate packaging. Intermec’s specially designed Duratran and Duratherm Block Out labels provide this important function, reducing costs and improving efficiency.
-
- Retail/Price Markdowns – When retail items need to be price-adjusted or product information must be modified, relabeling with a material that obscures the original information saves time and maintains the integrity of the original packaging.
-
- Pharmacy/Unit Level Dose – On prescription items, labels are commonly applied over original packaging for distribution to the customer/patient. It is often important that the original printing does not show through. For reliable retail barcode scanning, it is critical to eliminate interference from underlying codes.
-
- Warehouse/Logistics – In shipping and receiving operations, labels are sometimes applied to materials that frequently go through multiple use cycles. Shipping containers arriving in a facility can be reused to send materials to another facility by applying a new label over the existing label on the container. Previous information must not be visible through the newly applied label, especially in automated handling systems where it could interfere with routing of the container or carton.
Continue reading »
Blood Bag Labeling
Industry Need
With nearly 5 million Americans requiring blood transfusions annually, there is a tremendous need for blood products to supply these important therapies. Blood banks and collection centers require robust labels to reliably track blood from the donor to the final recipient; these labels need to survive multiple processing, testing, and storage steps through challenging environmental conditions. Intermec’s blood bag labeling product set enables durable, positive tracking of these critical components.
Bar coded blood bag labels enable the high degree of accuracy required for successful transfusion therapy. Clearly identified blood typing prevents serious complications that could result from donor type incompatibility. Different blood components and storage conditions yield varying shelf life; clear expiration date identification guarantees patients receive only safe, effective products. Formats configured in compliance with ISBT-128 standards provide interoperability with multiple collection centers, processing locations, and hospital systems.
In order for essential data to follow the contents of the blood bag, labels must endure a series of challenging conditions. Labels must maintain a strong bond to the flexible blood bag during centrifugation while whole blood is separated into components. Labels must remain positively attached during standard or cryogenic freezing (plasma and some red cells), refrigeration (red cells), or continuous shaking at room temperature (platelets). The bond must then be maintained during warming, which often includes immersion in a water bath.
Beyond the physical performance needs, blood bag labels must also meet regulatory requirements. The United States Food and Drug Administration mandates compliance with 21 CFR175.105 to increase safety should components of the adhesive migrate through the plastic into the blood bag.
Continue reading »
Bar Code Printing Options for Zebra Printers with Oracle WMS and MSCA
Executive Summary
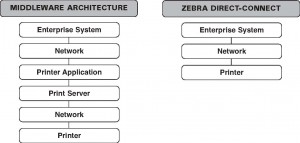
Bar code output from the Oracle E-Business Suite® environment is traditionally accomplished through third-party software. However, Oracle’s Warehouse Management System (WMS) and Mobile Supply Chain Applications (MSCA) offer a new approach that can simplify bar code label printing. Oracle WMS and MSCA produce output in XML data streams, instead of a proprietary Oracle format. Zebra Technologies has embedded an XML parser in its XML-enabled printers, so output from Oracle WMS and MSCA is natively understood by the printer without additional middleware or server hardware. The graphic below illustrates the system architectures and components required for bar code output from Oracle WMS and MSCA using the middleware and Zebra direct connection approaches.
This white paper describes the middleware and direct-connect bar code printing options for Oracle WMS and MSCA, explains the system requirements for each, and provides guidance as to when each approach is best suited to particular environments.